
PublicationsAPSR Endorsements, Position Papers and Guidelines
Molecular Testing of Metastatic Non-Small Cell Lung Cancer in the Asia-Pacific Region (APSR Lung Cancer Assembly, 2020)
POSITION STATEMENT of the LUNG CANCER ASSEMBLY (2020)
See also commentary: onlinelibrary.wiley.com/doi/10.1111/resp.13833
Authors:
1 CHONG-KIN LIAM MBBS, FRCP(UK), FCCP, FAPSR
2 EMILY STONE MBBS, MMed, FRACP
3 SITA ANDARINI MD, PhD
4 YONG-SHENG LIAM MBBS
5 DAVID CHI-LEUNG LAM MD, PhD, FRCP, FAPSR
6 PYNG LEE MBBS MD, FRCP(UK), FCCP, PhD
on behalf of the Lung Cancer Assembly of the APSR
1. Department of Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
2. Department of Thoracic Medicine, St. Vincent’s Hospital, Sydney, Australia
3. Department of Pulmonology and Respiratory Medicine, University of Indonesia-Persahabatan Hospital, Jakarta, Indonesia
4. Clinical Investigation Centre, University Malaya Medical Centre, Kuala Lumpur, Malaysia
5. Department of Medicine, University of Hong Kong, Hong Kong
6. Department of Medicine, National University Hospital, Singapore
Corresponding author:
Chong-Kin Liam
Department of Medicine,
Faculty of Medicine,
University of Malaya,
50603 Kuala Lumpur,
Malaysia
Fax Number: +603-79556936
Email address: liamck@ummc.edu.my
Abstract
The management of advanced non-small cell lung cancer (NSCLC) patients has become more complex with the identification of many oncogenic drivers starting with epidermal growth factor receptor (EGFR) mutation and the availability of therapies specifically targeting these molecular alterations resulting in improved treatment outcomes. Molecular biomarker testing of NSCLC is now considered standard of care and part of the diagnostic algorithm to identify subsets of patients for molecular-targeted treatment. With the increasing use of small biopsies and cytological specimens for diagnosis and the need to identify an increasing number of predictive biomarkers, proper management of the limited amount of sampling materials available is important. A limited diagnostic workup is recommended to preserve sufficient tissue for molecular testing. Molecular testing of lung adenocarcinoma for alterations in the EGFR, ALK and ROS1 genes is now considered standard of care while emerging predictive biomarkers such as mutations in BRAF, HER2, MET exon 14, RET, and NTRK may be included in an expanded panel testing. The extent of therapy-predictive biomarker testing is dependent on the approval and reimbursement status of the recommended therapeutic agents in each country or institution. When selecting a testing method, timeliness of results availability for treatment decision-making needs to be considered. Turnaround time should not be more than 10 working days from receipt of sample to reporting of all results. This position statement recommends molecular profiling of metastatic NSCLC at diagnosis of and at disease progression following first-line targeted therapy as this impacts on the treatment outcomes of patients.
Word count for abstract: 249 words
Word count for main text: 2,495 words
Key words: Asia-Pacific region, molecular testing, NSCLC
Short title: Molecular testing of metastatic NSCLC
List of abbreviations:
ALK, anaplastic lymphoma kinase; AMP, Association for Molecular Pathology; BRAF, B-Raf proto-oncogene; C797S, substitution of cysteine with serine at position 797 of exon 20; CAP, College of American Pathologists; cfDNA, cell-free plasma DNA; CPG, clinical practice guidelines; ctDNA, circulating tumour DNA; DNA, deoxyribonucleic acid; EBUS, endobronchial ultrasound; EGFR, epidermal growth factor receptor; ERBB2, Erb-B2 receptor tyrosine kinase-2; EUS, endoscopic ultrasound; ESMO, European Society of Medical Oncology; FDA, Food and Drug Administration; FFPE, formalin-fixed and paraffin-embedded; FISH, fluorescence in situ hybridisation; FNA, fine needle aspiration; fine needle aspiration (FNA); HER2, human epidermal growth factor receptor-2; IASLC, International Association for the Study of Lung Cancer; IHC, immunohistochemistry; KRAS, Kirsten rat sarcoma 2 viral oncogene homologue; MEK, mitogen-activated protein kinase kinase; MET, mesenchymal-epithelial transition factor; NCCN, National Comprehensive Cancer Network; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer; NTRK, neurotrophic receptor tyrosine kinase 1; PD-1, programmed cell death protein-1; PD-L1, programmed cell death ligand-1; PCR, polymerase chain reaction; ROS1, ROS proto-oncogene 1; RET, RET proto-oncogene; RT-PCR, real-time polymerase chain reaction; ROSE, rapid on-site evaluation; SCC, squamous cell carcinoma; T790M, substitution of threonine with methionine at position 790 of exon 20; TKI, tyrosine kinase inhibitor; TMB, tumour mutational burden; TTF-1, thyroid transcription factor 1; TTNB, transthoracic core needle biopsy.
INTRODUCTION
Lung cancer is the leading cause of cancer death for both men and women worldwide.1 Of an estimated 2.09 million new lung cancer cases worldwide annually, 58.5% occur in Asia and 60.7% of the 1.76 million annual lung cancer deaths worldwide occur in Asia.2 Non-small-cell lung cancer (NSCLC) accounts for about 85% of lung cancers.3 The majority of patients present with locally advanced or metastatic disease.1,4,5 In recent years, several somatic mutations that contribute to the carcinogenesis of NSCLC have been identified.3,6 Lung cancer in never smokers, which accounts for about a quarter of lung cancer in Asia,7 is considered a different disease with specific molecular and genetic differences from that of smokers.8 In never smokers with lung cancer, there is a higher prevalence of adenocarcinomas and women, especially Asian women.9
EGFR activating mutations, one of the most commonly mutated genes in NSCLC, are harboured by up to 15% of adenocarcinoma and less than 5% of squamous cell carcinomas (SCC) in Caucasian patients3,10 but are much more common in Asian populations, with prevalence rates reported in up to 59% of adenocarcinoma and 5.4% of SCC cases.11,12,13 EGFR mutations are also more common in never-smokers and female lung cancer patients.13,14 Improved progression-free survival, response rate, symptom control, and quality of life are observed in EGFR-mutant patients treated with an EGFR-tyrosine kinase inhibitor (TKI) compared with platinum doublet chemotherapy in many phase III clinical trials.11,15,16,17,18,19,20,21 EGFR mutations are now accepted as the predictive biomarker of treatment outcome to first-line EGFR-TKIs.22,23 In recent years, many more gene alterations that predict responsiveness to targeted therapy have been discovered in NSCLC and molecular testing has now expanded to include targets such as anaplastic lymphoma kinase (ALK) and ROS proto-oncogene 1 (ROS1) translocations, B-Raf proto-oncogene (BRAF) mutation, mesenchymal-epithelial transition (MET) amplification and mutation, RET proto-oncogene (RET) fusion, Erb-B2 receptor tyrosine kinase-2 (ERBB2) [or human epidermal growth factor receptor-2 (HER2)] mutation, neurotrophic receptor tyrosine kinase (NTRK) and Kirsten rat sarcoma 2 viral oncogene homologue (KRAS) mutation.24,25,26 In addition, immunotherapy has emerged as part of standard of care treatment for patients whose tumours do not harbour driver mutations. As such, the management of advanced NSCLC now requires testing for multiple biomarkers to guide the treatment strategy prompting the need to establish a consensus statement and recommendations for predictive biomarker testing and molecular profiling of metastatic NSCLC in the Asia-Pacific region. Although there are national practice guidelines for lung cancer treatment27,28 and an Asian adaptation of the European Society of Medical oncology (ESMO) guidelines for the management of metastatic NSCLC,25,29,30 there are no specific guidelines or recommendations on molecular testing of NSCLC for the region. Wide variations in testing are to be expected across the region because of variations in the availability of testing facility and reimbursement for both testing and lung cancer treatment in the healthcare systems of different countries.4,31
Purpose:
This position statement paper by the Lung Cancer Assembly of the Asian Pacific Society of Respirology (APSR) highlights important aspects of molecular and biomarker testing for patients with metastatic NSCLC in the Asia-Pacific region and is not a treatment guideline. In addition, immunotherapy with checkpoint inhibitors has emerged as part of standard of care treatment for advanced stage NSCLC patients.
METHODS
CKL, as the head of the APSR Lung Cancer Assembly, chaired a panel of respiratory physicians (except YSL) who were selected for their expertise in various aspects of lung cancer diagnosis and management. This position statement is the result of face-to-face discussions at APSR Congress meetings and exchange of views via email communications among these experts to develop the scope and draft the recommendations.
To understand the practice of EGFR mutation and other molecular testing in NSCLC across countries in the Asia-Pacific region the APSR membership was surveyed using a web-based online survey tool (SurveyMonkey) during the period 18 August to 3 October 2018.32 The survey questionnaire is as shown in Appendix A and the results are presented in Appendix B.
All authors reviewed and approved the final manuscript which is the outcome of the expert group discussion and consensus arrived on 5 December 2019. The panel did not receive any commercial sponsorship and all panel members worked on an honorary basis. This position paper is endorsed by the APSR and is posted at the APSR website as a resource document. The clinical relevance of the document will be reviewed after a maximum of 5 years.
RECOMMENDATIONS
The recommendations for molecular testing of metastatic NSCLC in this position statement are outlined in Table 4a and Table 4b which also show a comparison with the recommendations by other scientific societies.
Types of specimen suitable for molecular testing
Formalin-fixed paraffin-embedded (FFPE) core tissue biopsy is the preferred sample for molecular testing (Table 4a). However, cell blocks or any cytology sample including smear preparations with adequate cellularity and preservation may be tested for lung cancer molecular biomarkers.24
Specimen sampling procedures
The testing for predictive biomarkers prior to treatment initiation in patients with newly diagnosed metastatic NSCLC requires close coordination between the respiratory physician, interventional radiologist, pathologist, and oncologist to ensure that the specimen is used judiciously to obtain the required information for treatment decision-making.33 Sufficient tissue must be obtained to make a histological diagnosis of lung cancer and for molecular testing.34
Factors to be considered when choosing the optimal diagnostic approach include the anticipated diagnostic yield, diagnostic accuracy, adequacy of the amount of tissue specimen obtainable, invasiveness and risk of procedure, accessibility and timeliness of procedure, and technologies and expertise available. Patients with central lesions and suspected endobronchial involvement should undergo bronchoscopy30 (Table 4a) which allows endobronchial forceps biopsy, bronchial washing, bronchial brushing, as well as bronchial and transbronchial needle biopsy, with a diagnostic yield of 65%-88%.35,36,37 Patients with suspected hilar and mediastinal nodal disease should be biopsied by endobronchial ultrasound (EBUS) and/or endoscopic ultrasound (EUS), or mediastinoscopy.38 By combining direct bronchoscopic visualization of the airway and ultrasound-guided biopsy of the lesion, EBUS has a diagnostic yield of 75%-85% in large central lesions.39,40 Transthoracic core needle biopsy (TTNB) or fine needle aspiration (FNA) under CT imaging guidance is indicated in mid to peripheral lesions.25,30 Thoracoscopic evaluation and biopsy of the pleura should be considered if cytology results are negative on repeated thoracocenteses in patients with pleural effusions. Rapid on-site evaluation (ROSE) by the cytopathologists and/or cytotechnologists should be carried out when available to increase the yield at the time of specimen sampling which may reduce the need for additional diagnostic procedures.
When limited tissue is obtained, laboratories should use techniques to maximise the tissue, including “up-front” slide sectioning, for diagnostic and molecular biomarker testing.26 The use of immunohistochemistry (IHC) should be limited and molecular testing should be prioritised. In small biopsy specimens, a limited number of immunostains with one lung adenocarcinoma marker [thyroid transcription factor 1 (TTF-1) or napsin A] and one squamous carcinoma marker (p40 or p63) should suffice for diagnosis.26
A European expert group recommends41
- At least five endobronchial/transbronchial forceps biopsies with consideration for an additional five forceps biopsies or two cryobiopsies to maximise the amount of tissue obtained,
- At least four EBUS/EUS needle aspiration passes per target lesion, and
- At least two percutaneous core needle biopsies with an 18-20 gauge needle to ensure sufficient tissues while three to six core needle biopsies may be considered to maximise the amount of tissue obtained.
Molecular testing methods
Testing methods must be able to detect molecular alterations in specimens with as little as 20% cancer cells.24 Polymerase chain reaction (PCR)-based methods are more sensitive than Sanger sequencing and can reliably detect low frequency mutations in small samples.
EGFR testing methods should cover EGFR mutations in exons 18–21 (Table 4a).24,25,26 If resources or specimen material are limited, at least the most common activating mutations, i.e., exon 19 deletion and exon 21 L858R point mutation should be tested.24,25
For ALK testing, IHC with high-performance ALK antibodies and validated assays [e.g., FDA-approved IHC (ALK [D5F3] CDx Assay] is accepted as equivalent to fluorescence in situ hybridisation (FISH)24 with a shorter turnaround time.
Since ROS1 rearrangement is relatively rare and its occurrence is mutually exclusive with other oncogenic drivers including EGFR and ALK, sequential testing of EGFR and ALK followed by ROS1 is a pragmatic approach. If ROS1 IHC is used for screening, a positive result should be confirmed by FISH or real-time (RT)-PCR or next-generation sequencing (NGS). The high sensitivity of IHC means tumours that stain negative for ROS1 can be considered ROS1-negative.24
RT-PCR, Sanger sequencing (ideally paired with tumour enrichment) and NGS are the commonly used methods for testing BRAF mutation.26
When choosing a testing method, it is important to consider the timeliness of results availability. Turnaround time should not be more than 10 working days from sample receipt to reporting of all results.24,26
With the growing list of targetable gene alterations, there is increasing demand for multiplexed and simultaneous testing for many genes at once rather than sequential single-gene testing at the time of diagnosis. Compared to sequential single gene testing, NGS targeted gene panels with its ability to test multiple genes of interest on limited material and lower cancer cell concentration from small biopsies and cytological samples, offer a good option for rapid, cost-efficient and sample preserving genomic profiling.42 However, in most clinical settings sequential testing may be more cost effective. If available, NGS panels are preferred over single-gene assays for expanded testing beyond EGFR, ALK and ROS1.25,30
As shown in the results of our survey (Appendix B), the access to and the performance of predictive biomarker testing are generally driven by the availability, affordability and reimbursement status of tests and treatments.32 High volume practices are more likely to perform such tests.32 Given the diversity of healthcare infrastructure and economies, variations in practice, resource availability, healthcare financing and standards of care are to be expected across countries in the Asia-Pacific region. Oncology practice in lower income countries often lags behind the rest of the world. As much as practicing clinicians are enthusiastic about embracing evidence-based advances and recommendations by international guidelines, lower income countries face financial constraints and other challenges in adopting such recommendations.
Molecular testing at the initial diagnosis of metastatic NSCLC
Molecular testing are largely driven by the availability of targeted therapy (Table 4b). The recommended algorithm for molecular testing in metastatic NSCLC patients is shown in Figure 1. Since the vast majority of oncogene-addicted lung cancers are adenocarcinomas, all patients with metastatic, possible, probable or definite, adenocarcinoma should be tested for oncogenic drivers which include EGFR mutations, and ALK and ROS1 rearrangements.24,25,26 Although oncogenic drivers are much more common in adenocarcinomas in never-smokers (i.e., those who never smoked or who smoked < 100 cigarettes in lifetime), light-smokers (< 15 pack-years) or long-time ex-smokers (quitted > 10 years) they can also be found in current smokers and patients who smoke more heavily.
For squamous cell carcinoma, testing for EGFR mutations, and ALK and ROS1 rearrangements are recommended in never- or light-smokers or long-time ex-smoker, those with small biopsy specimens in which an adenocarcinoma component cannot be excluded, and those with adenosquamous carcinoma.34
Upfront testing should include EGFR mutations and rearrangements of ALK and ROS1. BRAF mutation testing maybe included as part of larger testing panels performed either initially or when routine EGFR, ALK, and ROS1 testing are negative and if BRAF/MEK inhibitors are available for use.24,26 Additional genetic testing of emerging biomarkers such as MET, HER2, RET and NTRK may be carried out if broader molecular profiling by NGS is performed24,25,26 to identify patients for treatment with specific inhibitors of these targets if such treatments are available or to direct patients to clinical trials. Individual testing for EGFR mutation, ALK and ROS1 can usually be done within 2 weeks while broader molecular profiling by NGS often takes 3 weeks or longer. In practice, the immediately druggable EGFR mutation, ALK and ROS1 are tested upfront for the timely commencement of appropriate first-line targeted therapy. If these three genetic alterations are not detected, first-line chemotherapy can be started while waiting for results of NGS testing for emerging biomarkers with potentially effective therapies.
Molecular testing in patients who develop disease progression on targeted therapy
Molecular testing for patients who develop disease progression on first-line and/or second-line targeted therapy should take into consideration the results of previous molecular testing and treatment received (Table 4b) (Figure 1)
It is strongly recommended that in patients with sensitizing EGFR-mutant lung adenocarcinoma who have progressed while on treatment with a first- or second-generation EGFR-TKI, acquired EGFR T790M exon 20 substitution mutation testing should be done to select them for therapy with osimertinib, a third-generation T790M-mutant specific EGFR-TKI.24,25,26
Peripheral blood for plasma cell-free DNA (cfDNA) or circulating tumour DNA testing is an acceptable approach to detect T790M at relapse but the test lacks sensitivity and there is a 20%-30% chance of missing T790M mutation.43 The presence of the original sensitising EGFR mutation in the liquid biopsy will serve as an indicator of sufficient cfDNA in the plasma sample. If plasma testing is negative for T790M, tumour tissue rebiopsy is strongly recommended to determine T790M status by tissue-based testing.24,25,26,44 If T790M mutation is not detected by a sensitive assay, testing for alternative mechanisms of resistance (MET amplification, HER2 amplification) may be carried out to direct the patients for therapies targeting these other molecular mechanisms of acquired resistance.26 A repeat tissue biopsy may also be more effective in identifying other resistance mechanisms which may require alternative treatment which include MET amplification, HER2 alterations, and small cell transformation.25
For patients with ALK rearranged NSCLC who have failed first-line treatment with crizotinib, routine identification of specific tyrosine kinase domain mutations is not recommended to determine the appropriate next line treatment,27 since most crizotinib-resistant tumours do not develop ALK resistance mutations and are sensitive to more potent second-generation ALK-TKIs. As newer-generation ALK-TKIs are increasingly being used in the first- and second-line settings, ALK mutation testing by plasma cfDNA or tumour rebiopsy may become a routine at relapse to select the next ALK-TKI as secondary ALK tyrosine kinase domain resistance mutations are more common after treatment with second-generation ALK-TKIs and because of the differential sensitivity to different second- or third-generation ALK-TKIs depending on the specific ALK tyrosine kinase resistance mutations.25,26,46,47
Biomarker testing for therapy with immune checkpoint inhibitors in patient with metastatic NSCLC
Programmed death-ligand 1 (PD-L1) expression on tumours by IHC should be determined before first-line treatment in patients with metastatic NSCLC without common driver mutations (Table 4b) (Figure 1).34 PD-L1 testing is required as a companion diagnostic for pembrolizumab monotherapy in all lines of treatment but not required for prescribing nivolumab or atezolizumab monotherapy in second-line although it may be informative as a complementary diagnostic.25,30
In different clinical trials, tissue- and blood-based tumour mutational burden (TMB) has been evaluated as a predictor of clinical benefit from immunotherapy with nivolumab, ipilimumab, atezolizumab and durvalumab but definitive cut-offs are yet to be defined.48,49,50,51
CONCLUDING STATEMENT
The recommendations in this position statement are summarised in Table 2. In the near future, more cost effective broad molecular testing with shorter turnaround time is anticipated to increase as more druggable biomarkers are identified and as technology advances.
Acknowledgements: The submission of this manuscript is made possible by funding from the APSR Assembly Fund.
Disclosure statement
| Name | Interest / Activity type | Entity | |
| 1 | Chong-Kin Liam | Research grants | AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme |
| Honoraria and fees for lectures and advisory board | AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, Pfizer, Takeda | ||
| 2 | Emily Stone | Nothing to declare | |
| 3 | Sita Andarini | Honoraria and fees for lectures, and advisory board meeting | AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Lilly, Mundipharma, Roche, Pfizer, Prodia, Takeda |
| 4 | Yong-Sheng Liam | Nothing to declare | |
| 5 | David Chi-Leung Lam | Research Grants | AstraZeneca, Boehringer Ingelheim |
| 6 | Pyng Lee | Nothing to declare |
REFERENCES
1. Latest global cancer data (2018). Available from: who.int/cancer/PRGlobocanFinal.pdf (Accessed 6 October 2019)
2. Globocan 2018 – Lung cancer [Internet]. Available from: gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf (Accessed 6 October 2019)
3. Dearden S, Stevens J, Wu Y-L, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann. Oncol. 2013; 24:2371-6.
4. Liam C-K, Andarini S, Lee P, Ho JC-M, Chau NQ, Tscheikuna J. Lung cancer staging now and in the future. Respirology 2015; 20:526-34.
5. Liam CK, Pang YK, Leow CH, Shyamala P, Menon AA. Changes in the distribution of lung cancer cell types and patient demography in a developing multiracial Asian country. Lung Cancer 2006; 53:23-30.
6. Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan MB, Fulton L, Fulton RS, Zhang Q, Wendl MC, Lawrence MS, Larson DE, Chen K, Dooling DJ, Sabo A, Hawes AC, Shen H, Jhangiani SN, Lewis LR, Hall O, Zhu Y, Mathew T, Ren Y, Yao J, Scherer SE, Clerc K, Metcalf GA, Ng B, Milosavljevic A, Gonzalez-Garay ML, Osborne JR, Meyer R, Shi X, Tang Y, Koboldt DC, Lin L, Abbott R, Miner TL, Pohl C, Fewell G, Haipek C, Schmidt H, Dunford-Shore BH, Kraja A, Crosby SD, Sawyer CS, Vickery T, Sander S, Robinson J, Winckler W, Baldwin J, Chirieac LR, Dutt A, Fennell T, Hanna M, Johnson BE, Onofrio RC, Thomas RK, Tonon G, Weir BA, Zhao X, Ziaugra L, Zody MC, Giordano T, Orringer MB, Roth JA, Spitz MR, Wistuba II, Ozenberger B, Good PJ, Chang AC, Beer DG, Watson MA, Ladanyi M, Broderick S, Yoshizawa A, Travis WD, Pao W, Province MA, Weinstock GM, Varmus HE, Gabriel SB, Lander ES, Gibbs RA, Meyerson M, Wilson RK. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 2008; 455(7216):1069-75.
7. Toh CK, Gao F, LimWT, Leong SS, Fong KW, Yap SP, Hsu AA, Eng P, Koong HN, Thirugnanam A, Tan EH. Never-smokers with lung cancer: epidemiologic evidence of a distinct disease entity. J. Clin. Oncol. 2006; 24:2245-51.
8. Couraud S, Souquet PJ, Paris C, Dô P, Doubre H, Pichon E, Dixmier A, Monnet I, Etienne-Mastroianni B, Vincent M, Trédaniel J, Perrichon M, Foucher P, Coudert B, Moro-Sibilot D, Dansin E, Labonne S, Missy P, Morin F, Blanché H, Zalcman G; French Cooperative Intergroup IFCT. BioCAST/IFCT-1002: epidemiological and molecular features of lung cancer in never-smokers. Eur. Respir. J. 2015; 45:1403-14.
9. Ha SY, Choi SJ, Cho JH, Choi HJ, Lee J, Jung K, Irwin D, Liu X, Lira ME, Mao M, Kim HK, Choi YS, Shim YM, Park WY, Choi YL, Kim J. Lung cancer in never-smoker Asian females is driven by oncogenic mutations, most often involving EGFR. Oncotarget 2015; 6:5465-74.
10. Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011; 12:175-80.
11. Mok TS, Wu Y-L, Thongprasert S, Yang C-H, Chu D-T, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009; 361:947-57.
12. Zhou W, Christiani DC. East meets West: ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin. J. Cancer. 2011; 30:287-92.
13. Liam C-K, Wahid MIA, Rajadurai P, Cheah Y-K, Ng TS-Y. Epidermal growth factor receptor mutations in lung adenocarcinoma in Malaysian patients. J. Thorac. Oncol. 2013; 8:766-72.
14. Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007; 98:1817-24.
15. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T; North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010; 362:2380-8.
16. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M; West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010; 11:121-8.
17. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, De Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Muñoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L; Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012; 13:239-46.
18. Zhou C, Wu Y-L, Chen G, Feng J, Liu X-Q, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Hu C, Luo Y, Chen L, Ye M, Huang J, Zhi X, Zhang Y, Xiu Q, Ma J, Zhang L, You C. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011; 12:735-42.
19. Wu Y-L, Zhou C, Liam C-K, Wu G, Liu X, Zhong Z, Lu S, Cheng Y, Han B, Chen L, Huang C, Qin S, Zhu Y, Pan H, Liang H, Li E, Jiang G, How SH, Fernando MC, Zhang Y, Xia F, Zuo Y. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann. Oncol. 2015; 26:1883-9.
20. Sequist LV, Yang JC-H, Yamamoto N, O’Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, Su WC, Bennouna J, Kato T, Gorbunova V, Lee KH, Shah R, Massey D, Zazulina V, Shahidi M, Schuler M. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013; 31:3327-34.
21. Wu Y-L, Zhou C, Hu C-P, Feng J, Lu S, Huang Y, Li W, Hou M, Shi JH, Lee KY, Xu CR, Massey D, Kim M, Shi Y, Geater SL. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014; 15:213-22.
22. Xue C, Hu Z, Jiang W, Zhao Y, Xu F, Huang Y, Zhao H, Wu J, Zhang Y, Zhao L, Zhang J, Chen L, Zhang L. National survey of the medical treatment status for non-small cell lung cancer (NSCLC) in China. Lung Cancer 2012; 77:371-5.
23. Yatabe Y, Kerr KM, Utomo A, Rajadurai P, Tran VK, Du X, Chou TY, Enriquez ML, Lee GK, Iqbal J, Shuangshoti S, Chung JH, Hagiwara K, Liang Z, Normanno N, Park K, Toyooka S, Tsai CM, Waring P, Zhang L, McCormack R, Ratcliffe M, Itoh Y, Sugeno M, Mok T. EGFR mutation testing practices within the Asia Pacific region: results of a multicenter diagnostic survey. J. Thorac. Oncol. 2015; 10:438-45.
24. Lindeman NI, Cagle PT, Aisner DL, Arcila ME, Beasley MB, Bernicker EH, Colasacco C, Dacic S, Hirsch FR, Kerr K, Kwiatkowski DJ, Ladanyi M, Nowak JA, Sholl L, Temple-Smolkin R, Solomon B, Souter LH, Thunnissen E, Tsao MS, Ventura CB, Wynes MW, Yatabe Y. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J. Thorac. Oncol. 2018; 13:323-58.
25. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, Peters S; ESMO Guidelines Committee. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018; 29: 1v192–iv237.
26. NCCN Guidelines: Non-Small Cell Lung Cancer. Version 1.2020, November 6, 2019. nccn.org/professionals/physician_gls/pdf/nscl.pdf (Accessed 6 December 2019)
27. The Japanese Lung Cancer Society. Lung Cancer Practice Guidelines Version 1.1; haigan.gr.jp/modules/guideline/index.php?content_id¼3 2017 (Accessed 6 October 2019).
28. Zhou Q, Wu YL. Developing CSCO lung cancer practice guidelines stratified by resource availability and treatment value. J. Glob. Oncol. 2016; 3:285-8.
29. Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, Kerr K, Popat S, Reck M, Senan S, Simo GV, Vansteenkiste J, Peters S; ESMO Guidelines Committee. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016; 27(Suppl 5): v1–v27.
30. Wu YL, Planchard D, Lu S, Sun, Yamamoto N, Kim DW, Tan DSW, Yang JCH, Azrif M, Mitsudomi T, Park K, Soo RA, Chang JWC, Alip A, Peters S, Douillard JY. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic non-small cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann. Oncol. 2019; 30:171-210.
31. Ellison G, Zhu G, Moulis A, Dearden S, Speake G, McCormack R. EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J. Clin. Pathol. 2013; 66:79-89.
32. Liam CK, Stone E, Andarini S. APSR membership survey of EGFR molecular testing of NSCLC in the Asia Pacific region [abstract]. 2019 World Conference on Lung Cancer, 7-10 September 2019, Barcelona, Spain. Poster No. 1521
33. Tsao MS, Hirsch FR, Yatabe Y. IASLC atlas of ALK and ROS1 testing in lung cancer. North Fort Myers, FL: Editorial Rx Press; 2016.
34. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, D’Amico TA, DeCamp MM, Dilling TJ, Dobelbower M, Doebele RC, Govindan R, Gubens MA, Hennon M, Horn L, Komaki R, Lackner RP, Lanuti M, Leal TA, Leisch LJ, Lilenbaum R, Lin J, Loo BW Jr, Martins R, Otterson GA, Reckamp K, Riely GJ, Schild SE, Shapiro TA, Stevenson J, Swanson SJ, Tauer K, Yang SC, Gregory K, Hughes M. Non-small cell lung cancer, version 5.2017. NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2017; 15:504-35.
35. Ost DE, Ernst A, Lei X, Kovitz KL, Benzaquen S, Diaz-Mendoza J, Greenhill S, Toth J, Feller-Kopman D, Puchalski J, Baram D, Karunakara R, Jimenez CA, Filner JJ, Morice RC, Eapen GA, Michaud GC, Estrada-Y-Martin RM, Rafeq S, Grosu HB, Ray C, Gilbert CR, Yarmus LB, Simoff M; AQuIRE Bronchoscopy Registry. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. Results of the AQuIRE Registry. Am. J. Respir. Crit. Care Med. 2016; 193: 68-77.
36. Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e142S-e165S.
37. van der Drift MA, van der Wilt GJ, Thunnissen FB, Janssen JP. A prospective study of the timing and cost-effectiveness of bronchial washing during bronchoscopy for pulmonary malignant tumors. Chest 2005; 128:394-400.
38. Adams K, Shah PL, Edmonds L, Lim E. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax 2009; 64:757-62.
39. Herth F, Becker HD, Ernst A. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest 2004; 125: 322-5.
40. Paone G, Nicastri E, Lucantoni G, Dello Iacono R, Battistoni P, D’Angeli AL, Galluccio G. Endobronchial ultrasound-driven biopsy in the diagnosis of peripheral lung lesions. Chest 2005; 128:3551-7.
41. Dietel M, Bubendorf L, Dingemans AC, Dooms C, Elmberger G, García RC, Kerr KM, Lim E, López-Ríos F, Thunnissen E, Van Schil PE, von Laffert M. Diagnostic procedures for non-small-cell lung cancer (NSCLC): recommendations of the European Expert Group. Thorax 2016; 71:177-84.
42. Khoo C, Rogers TM, Fellowes A, Bell A, Fox S. Molecular methods for somatic mutation testing in lung adenocarcinoma: EGFR and beyond. Transl. Lung Cancer Res. 2015; 4:126-141.
43. Sundaresan TK, Sequist LV, Heymach JV, Riely GJ, Jänne PA, Koch WH, Sullivan JP, Fox DB, Maher R, Muzikansky A, Webb A, Tran HT, Giri U, Fleisher M, Yu HA, Wei W, Johnson BE, Barber TA, Walsh JR, Engelman JA, Stott SL, Kapur R, Maheswaran S, Toner M, Haber DA. Detection of T790M, the acquired resistance EGFR mutation, by tumor biopsy versus noninvasive blood-based analyses. Clin. Cancer Res. 2016; 22:1103-10.
44. Mok T, Wu YL, Lee JS, Yu CJ, Sriuranpong V, Sandoval-Tan J, Ladrera G, Thongprasert S, Srimuninnimit V, Liao M, Zhu Y, Zhou C, Fuerte F, Margono B, Wen W, Tsai J, Truman M, Klughammer B, Shames DS, Wu L. Detection and dynamic changes of EGFR mutations from circulating tumor DNA as a predictor of survival outcomes in NSCLC patients treated with first-line intercalated erlotinib and chemotherapy. Clin. Cancer Res. 2015; 21:3196-203.
45. Tan CS, Kumarakulasinghe NB, Huang YQ, Ang YLE, Choo JRE, Goh BC, Soo RA. Third generation EGFR TKIs: Current data and future directions. Mol. Cancer 2018; 17:29 doi.org/10.1186/s12943-018-0778-0.
46. Gainor JF, Dardaei L, Yoda S, Friboulet L, Leshchiner I, Katayama R, Dagogo-Jack I, Gadgeel S, Schultz K, Singh M, Chin E, Parks M, Lee D, DiCecca RH, Lockerman E, Huynh T, Logan J, Ritterhouse LL, Le LP, Muniappan A, Digumarthy S, Channick C, Keyes C, Getz G, Dias-Santagata D, Heist RS, Lennerz J, Sequist LV, Benes CH, Iafrate AJ, Mino-Kenudson M, Engelman JA, Shaw AT. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016; 6:1118-33.
47. Shaw AT, Solomon BJ, Besse B, Bauer TM, Lin CC, Soo RA, Riely GJ, Ou SHI, Clancy JS, Li S, Abbattista A, Thurm H, Satouchi M, Camidge R, Kao S, Chiari R, Gadgeel SM, Felip E, Martini JF. Lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J. Clin. Oncol. 2019; 37:1370-1379.
48. Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, Rittmeyer A, Fehrenbacher L, Otto G, Malboeuf C, Lieber DS, Lipson D, Silterra J, Amler L, Riehl T, Cummings CA, Hegde PS, Sandler A, Ballinger M, Fabrizio D, Mok T, Shames DS. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med. 2018; 24:1441-8.
49. Velcheti V, Kim ES, Mekhail T, C Dakhil, PJ Stella, X Shen, S Hu, SM Paul, DS Shames, C Yun, S-C Phan, MA Socinski. Prospective clinical evaluation of blood-based tumor mutational burden (bTMB) as a predictive biomarker for atezolizumab (atezo) in 1L non-small cell lung cancer (NSCLC): interim B-F1RST results. J. Clin. Oncol. 2018; 36:12001-12001.
50. Hellmann MD, Ciuleanu T-E, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, Borghaei H, Ramalingam SS, Brahmer J, Reck M, O’Byrne KJ, Geese WJ, Green G, Chang H, Szustakowski J, Bhagavatheeswaran P, Healey D, Fu Y, Nathan F, Paz-Ares L. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N. Engl. J. Med. 2018; 378: 2093-104.
51. Peters S, Cho BC, Reinmuth N, et al. Tumor mutational burden (TMB) as a biomarker of survival in metastatic non-small cell lung cancer (mNSCLC): Blood and tissue TMB analysis from MYSTIC, a Phase III study of first-line durvalumab ± tremelimumab vs chemotherapy [abstract]. Presented at: 2019 AACR Annual Meeting; March 29-April 3, 2019; Atlanta, GA. Abstract CT074.
Appendix A
Survey Questions on EGFR mutation and other molecular testing of NSCLC in the Asia-Pacific Region for APSR Membership
| Question | Format of response | |
| 1 | Country of practice? | Dropdown |
| 2 | Clinical specialty? | Dropdown ・Respiratory medicine ・Surgery ・Pathology ・Medical oncology ・Radiation oncology ・Radiology/Imaging ・Other (free text) |
| 3 | Primary work environment? | Dropdown ・Academic/tertiary centre ・Public hospital ・Private hospital ・Other |
| 4 | Proportion of NSCLC tested for EGFR mutation? | Percent of cases
Don’t know |
| 5 | Type of sample(s) most commonly tested? | Multiple choice (more than one answer permitted) ・tissue biopsy ・cytology specimen ・EBUS TBNA ・Liquid biopsy ・Other ・Don’t know |
| 6 | Method of EGFR mutation testing is most commonly used in your institution?* | DNA sequencing
Therascreen EGFR RGQ PCR KIT (qiagen) EGFR29 Mutation detection kit (AMOY) PNAClamp Mutation detection kit (Panagene) Other commercial in vitro diagnostic kit PNA-LNA PCR clamp PCR INVADER Cycleave Nest-generation sequencing Other laboratory-developed method Don’t know |
| 7 | Cost of EGFR mutation testing? | a.In USD b.Range c.Cost for each test d.Cheapest/most expensive b. Don’t know |
| 8 | Is EGFR mutation reimbursed in your country? | Yes
No Partially Don’t know |
| 9 | Average time to test result from biopsy? | In days
Don’t know |
| 10 | Frequency of retesting after progression? | In percent
Don’t know |
| 11 | When do you test for T790M? | At initial diagnosis
At progression Don’t know May change after approval of osimertinib |
| 12 | On which sample do you test for T790M most often? | Rebiopsy of tissue
Liquid biopsy Don’t know |
| 13 | Which drugs have been approved in your country | Erlotinib
Gefitinib Afatinib Osimertinib Other – please specify (e.g, icotinib in China, olmutinib in Republic of Korea) |
| 14 | How common is reflex testing | Reflex Physician initiated |
| 15 | Other molecular markers tested | No
Yes (select all that apply) ALK ROS 1 PD-L1 BRAF HER2 cMET |
*Testing options based on Yatabe Y, Kerr KM, Utomo A, et al. EGFR mutation testing practices within the Asia Pacific region: results of a multicenter diagnostic survey. J Thorac Oncol. 2015; 10:438-445.
Appendix B
Results of APSR membership survey on EGFR mutation and other molecular testing of NSCLC in the Asia-Pacific Region
Method
The survey was circulated as a web-based electronic online survey questionnaire (www.surveymonkey.com) from 18 August to 3 October 2018 to the entire membership of the Asian Pacific Society of Respirology. The study was approved by the St Vincent’s Hospital Human Research Ethics Committee (HREC File No. 15/252). Survey questions sought information on the following aspects of EGFR molecular testing: prevalence, methods of testing, funding and cost, type of tissue or sample used, time frame for test results, retesting after progression, prevalence of T790M testing, the use of liquid biopsy and the testing of other molecular alterations. The survey questions are shown in Appendix A.
Results of APSR membership survey
Respondents
Of the 129 respondents to this survey, 113 (87.6%) were respiratory physicians with a small number of respondents from medical oncology, thoracic surgery, pathology, clinical oncology, radiation oncology and family practice. The respondents were from 16 countries in the Asia-Pacific region with the largest number of respondents from Japan (40, 31%) followed by Malaysia (16, 12.4%), India (15, 11.6%) and Indonesia (14, 10.8%) with the remaining 44 respondents (34.1%) from the other 12 countries. A list of respondents per country is shown in Table 1. Of the 129 respondents, 119 (92.2%) treated lung cancer patients regularly with most (73, 61.3%) seeing fewer than 10 lung cancer patients per week, 38 (31.9%) seeing 10 to 30 lung cancer patients per week and 7 (5.9%) seeing more than 30 lung cancer patients per week. Of the 129 respondents, 60 (46.5%) were working in academic/tertiary centres, 46 (35.7%) in public hospitals and 23 (17.8%) in private hospitals.
Table 1 Number of respondents according to the Asia-Pacific countries
| Country | No. of respondents |
| Australia | 1 |
| Bangladesh | 8 |
| China | 9 |
| Hong Kong | 8 |
| India | 15 |
| Indonesia | 14 |
| Japan | 40 |
| Malaysia | 16 |
| Myanmar | 1 |
| Nepal | 2 |
| Philippines | 7 |
| Republic of Korea | 1 |
| Singapore | 2 |
| Sri Lanka | 3 |
| Thailand | 1 |
| Vietnam | 1 |
| Total | 129 |
Details of EGFR mutation testing
Of the 121 respondents who responded to the question on the percentage of lung cancer patients tested for EGFR mutation, over half (68, 56.2%) performed the test in more than 80% of the patients, 25 (20.7%) performed the test in 50% to 80% of the patients, 21 (17.4%) in less than 50% of the patients and only a few (7, 5.8%) who never ordered EGFR mutation testing. According to the 122 respondents who ordered EGFR mutation testing, almost all (116, 95.1%) EGFR mutation testing was performed on tissue biopsy specimens, while the test was performed less frequently on cytology specimens (83, 68.0%) and on plasma (liquid biopsy) (23, 18.9%). Over half of the 129 respondents (72, 55.8%) knew the most commonly used method for EGFR mutation testing and allele-specific RT-PCR was the most commonly identified testing method (51 of 72 respondents, 70.8%) followed by DNA sequencing (11 respondents, 15.2%). Three respondents selected next-generation sequencing as the most commonly used method. The turn-around time for results of EGFR mutation testing most commonly fell between 7-14 days (72 of 126 respondents, 57.1%) but could be less than 7 days (29, 23.0%) or more than 14 days (25, 19.8%).
Testing for molecular aberrations in the initial biopsy was more commonly physician initiated [89 of 121 respondents (73.5%)] than reflex (i.e., ordered by the reporting pathologist based on histopathology) [32 respondents (26.4%)].
Factors associated with higher rates of EGFR mutation testing
A number of factors were associated with higher rates of EGFR mutation testing among the respondents, including type of practice, number of lung cancer patients seen weekly and level of EGFR mutation test reimbursement. These results are summarised in Table 2.
Table 2 Factors associated with higher rates of EGFR mutation testing among APSR members
| Factor | Testing rates | OR (95% CI), p value |
| Academic/tertiary/public vs. private hospital | 96/99 (97%) vs. 18/22 (81.8%)* | 7.11 (1.47-34.5), p=0.02 |
| ≥10 patients vs. < 10 patients seen weekly | 40/45 (88.9%) vs. 49/71 (69.0%)** | 3.56 (1.13-11.17), p=0.023 |
| Test fully reimbursed¶ vs. not fully reimbursed¶¶ | 46/51 (90.2%) vs. 47/70 (67.1%)** | 1.63 (1.25-2.12), p=0.003 |
*Reflects whether all patients were tested
**Reflects whether ≥50% patients tested
¶Full reimbursement by health insurance or sponsored by pharmaceutical company
¶¶Included partial reimbursement by health insurance or pharmaceutical company, no reimbursement at all, or respondent did not know
A significantly higher percentage of NSCLC patients was tested for EGFR mutation in academic/tertiary centres and public hospitals than in private hospitals [96 of 99 (97.0%)] vs [18 of 22 (81.8%)] (OR, 7.11; 95% CI, 1.47–34.50; p=0.02).
The percentage of EGFR mutation testing for >50% of cases was significantly higher when the number of lung cancer patients seen per week was ≥10 versus <10. [40 of 45 (88.9%)] vs [49 of 71 (69.0%)] (OR, 3.56; 95% CI, 1.13–11.17; p=0.023).
The percentage of EGFR mutation testing for >50% of cases was significantly higher when the test was fully reimbursed [46 of 51 (90.2%) compared to otherwise [47 of 70 (67.1%)] (OR, 1.63; 95% CI, 1.25–2.12; p=0.003).
Survey respondents also indicated which EGFR TKIs were approved or accessible for use in their practice. Gefitinib was approved in all countries surveyed, erlotinib in all except Sri Lanka, afatinib in all except Bangladesh, Myanmar, Sri Lanka and Thailand, and osmertinib in all except Bangladesh, Myanmar, Nepal, Sri Lanka and Thailand. Accessibility of osimertinib was associated with higher rates of tissue rebiopsy at disease progression on 1st or 2nd generation EGFR TKI and with availability of liquid biopsy for T790M mutation detection (Table 3).
A significantly higher percentage of respondents would perform tissue rebiopsy in >50% of the cases with disease progression while on treatment with first- or second-generation EGFR TKI if osimertinib was accessible for use [34 of 72 (47.2%) compared to 7 of 49 (14.3%) if otherwise (OR, 5.37; 95% CI, 2.13-13.53; p<0.0001). Availability of liquid biopsy for T790M resistance mutation detection was higher in practices where there was access to osimertinib (91.6% vs 28.6%; OR, 27.50, 95% CI, 9.72-77.84; p<0.0001).
Table 3 Performance of tissue rebiopsy and liquid biopsy for T790M mutation according to availability of osimertinib
| Test performed at disease progression on first- or second-generation EGFR TKI | Osimertinib available vs. not available* | OR (95% CI), p value |
| Tissue rebiopsy | 34/72 (47.2%) vs. 7/49 (14.3%) | 5.37 (2.13-13.53, p<0.0001) |
| Availability of liquid biopsy for T790M mutation | 66/72 (91.6%) vs. 14/49 (28.6%) | 27.5 (9.72-77.84), p<0.0001 |
*Denominator = No. of respondents in each category
Other biomarkers were tested for more frequently when EGFR mutation testing was carried out for > 80% of cases, in particular testing for ALK rearrangement was significantly higher in this context [36 of 68 (52.9%)] vs [18 of 53 (34.0%); OR, 2.19 (95% CI, 1.04-4.59); p=0037].
Conclusions from the survey findings on EGFR mutation and other biomarker testing in the Asia-Pacific Region
This survey of the APRS membership identified some key features of EGFR mutation testing and to a lesser extent other biomarker testing for the region. Conclusions from the survey are limited by the small number of respondents but provide some important insights. EGFR mutation testing is widespread, APSR members are relatively knowledgeable about testing, which is more common in academic/tertiary and public centres. Respondents from higher volume centres more frequently perform EGFR mutation testing and rates of testing is influenced by levels of reimbursement for the test. First-generation EGFR TKIs are available fairly widely in the region while availability of later generation EGFR TKIs is more limited. Testing for T790M resistance mutation at disease progression is less common in areas where osimertinib is not available. Testing for EGFR mutation is associated with more frequent testing for other biomarkers.
Table 4a Outline of recommendations for molecular testing of metastatic NSCLC and comparison with recommendations by other scientific societies (Part 1)
| Society | Asian Pacific Society of Respirology Lung Cancer Assembly | College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology (CAP/IASLC/AMP)27 | National Comprehensive Cancer Network (NCCN)29 | European Society of Medical Oncology (ESMO)28 | Chinese Society of Clinical Oncology (CSCO)- European Society of Medical Oncology (ESMO)33 |
| Country/Region | Asia-Pacific | USA | USA | Europe | Asia |
| Expert panel members | Respiratory physicians with lung cancer expertise | Pathologists and oncologists with lung cancer expertise | Experts from 27 NCCN member institutions | Multidisciplinary experts in lung cancer | Panel of experts (2 each from the oncological societies of Japan (JSMO), Republic of Korea, (KMSO), Malaysia (MOS), Singapore (SSO) and Taiwan (TOS)* |
| Update schedule | The first position statement | First published 2013 Updated 2018 |
With each new FDA approved intervention | 2 yearly More frequent online updates |
The first guideline |
| Recommended specimen type | FFPE core tissue biopsy; cytology samples including cell blocks | FFPE tissue biopsy; cytology samples including cell blocks | FFPE material suitable for most molecular analyses, except bone biopsies treated with acid decalcifying solutions; cytopathology specimens including cell blocks, direct smears or touch preparations | FFPE tissue biopsy; cytology samples including cell blocks | FFPE tissue biopsy; cytology samples including cell blocks |
| Specimen sampling rocedures | Bronchoscopy for central or endobronchial lesions
Image-guided TTNB/TTNA for peripheral lesions EBUS, EUS or mediastinoscopy for suspected nodal disease Thoracocentesis for cytopathology and/or biopsy by thoracoscopy for pleural effusion Rapid on-site evaluation may increase the yield at the time of specimen sampling and reduce the need for additional diagnostic procedures |
Not covered | Bronchoscopy for central or endobronchial lesions
Navigational bronchoscopy, radial EBUS or TTNA for peripheral (outer one-third) nodules EBUS, EUS, navigational bronchoscopy or mediastinoscopy for suspected nodal disease Thoracocentesis and/or thoracoscopy for pleural effusion |
Bronchoscopy for central lesions
Image-guided TTNA for mid to peripheral lesions. EBUS and/or EUS for regional lymph nodes Thoracocentesis for pleural effusion Surgical approaches (mediastinoscopy, mediastinotomy, thoracoscopy, etc.) when less invasive techniques not diagnostic Constant communication between pathologists and procedure performers improve diagnostic yields. |
Similar recommendations as ESMO CPG |
| Molecular testing methods | EGFR mutations A validated EGFR mutation test method that covers common mutations in exons 18–21. When resources or material are limited, prioritise testing for exon 19 deletion and exon 21 L858R point mutation. |
EGFR mutations Prioritise EGFR mutations in exons 18–21. At a minimum, exon 19 deletion and exon 21 L858R point mutation should be determined. |
EGFR mutations Similar recommendation for EGFR mutation testing. |
EGFR mutations Prioritise EGFR mutations in exons 18–21 (especially exon 19 deletion and exon 21 L858R point mutation). |
Similar recommendations as ESMO CPG. |
| ALK rearrangement A validated IHC assay is equivalent to FISH. |
ALK rearrangement Recommends using FISH; IHC is an equivalent alternative. |
ALK rearrangement FDA-approved IHC (ALK [D5F3] CDx Assay) can be used without confirmation by FISH although confirmation by FISH is recommended. An appropriately designed and validated NGS platform can be used. |
ALK rearrangement Detection by FISH remains a standard. IHC with high-performance ALK antibodies and validated assays are an equivalent alternative. |
||
| ROS1 rearrangement ROS1 IHC may be used for screening with confirmation of positive results by FISH or RT-PCR or NGS. |
ROS1 rearrangement ROS-1 IHC testing has high sensitivity; accept negative results; confirm positive results with by FISH or RT-PCR or NGS. |
ROS1 rearrangement Testing may be performed using FISH, a validated NGS platform, or IHC which requires confirmation of positives because of a low specificity. |
ROS1 rearrangement Detection by FISH is standard with validated RT-PCR test an alternative. IHC may be used for screening because of its high sensitivity but its specificity is low. |
||
| Turnaround time Aim for results to be available within 10 working days of sample receipt. |
Turnaround time Should not be more than 10 days from sample receipt. |
||||
| Testing beyond EGFR, ALK and ROS1 Multiplexed genetic sequencing panels (e.g. NGS) preferred over multiple single-gene tests for treatment options beyond EGFR, ALK and ROS1. However, single gene assays are still acceptable. |
Testing beyond EGFR, ALK and ROS1 Multiplexed genetic sequencing panels preferred over multiple single-gene tests for treatment options beyond EGFR, ALK and ROS1. |
Testing beyond EGFR, ALK and ROS1 Broader molecular profiling to assess for emerging biomarkers. |
If available, multiplex platforms (e.g., NGS) are preferred. |
*CSCO, Chinese Society of Clinical Oncology; JSMO, Japanese Society of Medical Oncology; Korean Society of Medical Oncology; MOS, Malaysian Oncological Society; SSO, Singapore Society of Oncology; TOS, Taiwan Oncology Society
Abbreviations: ALK, anaplastic lymphoma kinase; BRAF, B-Raf proto-oncogene; C797S, substitution of cysteine with serine at position 797 of exon 20; CPG, clinical practice guidelines; cfDNA, cell-free DNA; EBUS, EGFR, epidermal growth factor receptor; EBUS, endobronchial ultrasound; EUS, endoscopic ultrasound; FDA, Food and Drug Administration; FFPE, Formalin-fixed paraffin embedded tissue; FISH, fluorescence in situ hybridisation; FNAC, fine needle aspiration cytology; IHC, immunohistochemistry; MEK, mitogen-activated protein kinase kinase; MET, mesenchymal-epithelial transition factor; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer; PD-1, programmed cell death protein-1; PD-L1, programmed cell death ligand-1; PCR, polymerase chain reaction; ROS1, ROS proto-oncogene 1; RET, RET proto-oncogene; RT-PCR, real-time polymerase chain reaction; SCC, squamous cell carcinoma; SCLC, small cell lung cancer; T790M; substitution of threonine with methionine at position 790 of exon 20; TKI, tyrosine kinase inhibitor; TMB, tumour mutational burden; TTNA, transthoracic needle aspiration; TTNB, transthoracic core needle biopsy
Table 4b Outline of recommendations for molecular testing of metastatic NSCLC and comparison with recommendations by other scientific societies (Part 2)
| Society | Asian Pacific Society of Respirology Lung Cancer Assembly | College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology (CAP/IASLC/AMP)27 | National Comprehensive Cancer Network (NCCN)29 | European Society of Medical Oncology (ESMO)28 | Chinese Society of Clinical Oncology (CSCO)- European Society of Medical Oncology (ESMO)33 |
| Molecular testing at initial diagnosis | Extent of molecular profiling is determined by drug availability and local reimbursement status.
All metastatic adenocarcinoma and non-squamous NSCLC should be tested for EGFR mutations, and ALK and ROS1 rearrangements. For SCC, testing for EGFR mutations, and ALK and ROS1 rearrangements are recommended in never- or light smokers or long-time ex-smoker, small biopsies, and adenosquamous carcinoma. The immediately druggable EGFR mutation, and ALK and ROS1 rearrangements are tested upfront. BRAF V600 mutation testing maybe part of larger testing panels performed initially or when EGFR, ALK, and ROS1 testing are negative to select for BRAF/MEK inhibitor use. Emerging biomarkers (e.g. MET, HER2, RET and NTRK1 gene alterations) may be tested if NGS is performed and if adequate material is available to direct patients to clinical trials. Consider first-line chemotherapy while waiting for results of emerging rare genetic alterations (e.g. BRAF V600E mutation, MET amplification, MET exon 14 skipping mutation). |
Similar recommendations for testing in adenocarcinoma and SCC.
“Must-test” biomarkers are EGFR mutation, and ALK and ROS1 rearrangements. “Should-test” new and emerging actionable biomarkers (BRAF, MET, RET, HER2 and KRAS) to direct patients to clinical trials should be included in large sequencing panels if adequate material is available; but not required if laboratory performs only single-gene assays. |
Test for sensitising EGFR mutations, BRAF V600E point mutations, ALK rearrangement, ROS1 rearrangement in advanced or metastatic non-squamous NSCLC (adenocarcinoma, large cell carcinoma and NSCLC-not otherwise specified)
For advanced or metastatic SCC, consider molecular testing for EGFR mutation and ALK in never smokers or small biopsy specimens, or mixed histology; and consider ROS1 and BRAF testing in small biopsy specimens or mixed histology Broad molecular profiling to identify emerging rare driver mutations (HER2 mutations, RET rearrangements, high-level MET amplification, MET exon 14 skipping mutations) if effective drugs available or to direct to clinical trials. |
Testing for EGFR mutations and ALK and ROS1 rearrangements in advanced non-squamous NSCLC are mandatory in most European countries.
BRAF V600E mutations testing in advanced non-squamous NSCLC for the prescription of BRAF/MEK inhibitors (increasingly approved in Europe). EGFR and ALK testing not recommended if SCC is confidently diagnosed except in never- (never smoked or who smoked <100 cigarettes in lifetime) or light (<15 pack-years) smokers or long-time ex-smoker (>10 years). HER2 and MET exon 14 mutations, and RET and NTRK1 gene fusions are considered evolving biomarkers. |
Similar recommendations as ESMO CPG. |
| Molecular testing at disease progression on first-line targeted therapy | To take into account previous molecular testing results and therapies prescribed. | ||||
| EGFR-mutant NSCLC treated with first-/second-generation EGFR-TKI T790M testing is essential to select for therapy with third-generation EGFR-TKI, osimertinib.. Plasma cfDNA testing is acceptable for detecting T790M mutation. If negative, tissue rebiopsy is strongly recommended for testing T790M and other genetic alterations (e.g. MET amplification, HER2 mutation) and histological transformation to SCLC. EGFR-mutant NSCLC treated with third-generation EGFR-TKI Plasma cfDNA testing for C797S mutation may be considered. Repeat tissue biopsy for more extensive NGS panel testing e.g. MET amplification, and SCLC transformation. |
EGFR-mutant NSCLC treated with first-/second-generation EGFR-TKI Strong recommendation for EGFR T790M exon 20 substitution mutation testing to select for third-generation EGFR-targeted therapy. Plasma cfDNA methods can be used to identify EGFR T790M; testing of the tumour sample is recommended if plasma result is negative. |
EGFR-mutant NSCLC treated with first-/second-generation EGFR-TKI At the minimum, testing forT790M to direct patients to third-generation EGFR-TKI therapy. With negative T790M, testing for alternative mechanisms of resistance (MET amplification, HER2 amplification) may be used to direct patients for additional therapies. |
EGFR-mutant NSCLC treated with first-/second-generation EGFR-TKI Mandatory to test for T790M because of availability of third-generation EGFR-TKI effective against T790M-mutant recurrent disease. Plasma cfDNA testing is acceptable for T790M detection but lacks sensitivity. If negative, T790M testing on tissue biopsy is strongly recommended. Tissue biopsy is more effective to detect other resistance mechanisms (e.g. SCLC transformation, MET amplification, HER2 alterations, etc.). |
EGFR-mutant NSCLC treated with first-/second-generation EGFR-TKI Testing for exon 20 T790M mutation on either re‑biopsy or cell block of FNAC or pleural effusion specimen or ctDNA should be considered Exclude SCLC transformation on re‑biopsy specimen. If feasible, analyse re-biopsy or cell block of FNAC or pleural effusion specimen for HER 2 mutation/amplification and MET amplification. |
|
| ALK-positive NSCLC treated with first-line crizotinib Testing preferable but not required. ALK-positive NSCLC treated with first-line newer generation ALK-TKI |
ALK-positive NSCLC Insufficient evidence to recommend for or against routine testing for ALK mutation status after treatment with an ALK-TKI. |
ALK-positive NSCLC It is unclear whether identiifcation of specific tyrosine kinase domain mutations can identify appropriate next steps in therapy, although preliminary data suggest that specific kinase domain mutations can impact next line of therapy. For patients who progress on first-line alectinib or ceritinib, molecular testing of a biopsy to review the resistance mechanism may be beneficial to determine the role of other ALK-TKIs. |
ALK-positive NSCLC Testing for ALK mutations as resistance mechanisms to ALK-TKIs may become routine as newer-generation ALK-TKIs show differential efficacy against different ALK mutations. |
ALK-positive NSCLC Assessment of the molecular mechanisms of resistance could impact treatment decision. Testing for ALK mutations as resistance mechanisms to ALK-TKIs may become routine as newer-generation ALK-TKIs show differential efficacy against different ALK mutations. |
|
| Biomarker testing to select patients for immunotherapy | PD-L1 testing by IHC may be performed on FFPE tissue samples on a validated platform.
PD-L1 testing is recommended if tumour is negative for common oncogenic drivers (EGFR, ALK, ROS1, BRAF gene alterations). PD-L1 testing is required for pembrolizumab therapy in all lines of treatment; not required but maybe informative when nivolumab or atezolizumab are prescribed as monotherapy in the second-line setting. |
IHC for PD-L1 is appropriate in many settings although exact testing protocols may vary for different treatments.
The potential utility of TMB calculations (mutations/base pair of total genomic sequence) as assessed by NGS panels is currently investigational. |
IHC for PD-L1 can be used to identify disease most likely to respond to first-line anti-PD-1/PD-L1. | PD-L1 expression testing by IHC is recommended for all patients with newly diagnosed advanced NSCLC.
PD-L1 testing is required for pembrolizumab therapy; not required but maybe informative when nivolumab or atezolizumab are used. No recommendation for or against the use of TMB as predictive biomarker. |
PD-L1 testing by IHC is recommended at diagnosis to inform the use of pembrolizumab in the first-line or second-line setting and maybe informative when nivolumab or atezolizumab are used as monotherapy in the second-line therapy setting.
No recommendation for or against the use of TMB as predictive biomarker. |
*CSCO, Chinese Society of Clinical Oncology; JSMO, Japanese Society of Medical Oncology; Korean Society of Medical Oncology; MOS, Malaysian Oncological Society; SSO, Singapore Society of Oncology; TOS, Taiwan Oncology Society
Abbreviations: ALK, anaplastic lymphoma kinase; BRAF, B-Raf proto-oncogene; C797S, substitution of cysteine with serine at position 797 of exon 20; CPG, clinical practice guidelines; cfDNA, cell-free DNA; EBUS, EGFR, epidermal growth factor receptor; EBUS, endobronchial ultrasound; EUS, endoscopic ultrasound; FDA, Food and Drug Administration; FFPE, Formalin-fixed paraffin embedded tissue; FISH, fluorescence in situ hybridisation; FNAC, fine needle aspiration cytology; IHC, immunohistochemistry; MEK, mitogen-activated protein kinase kinase; MET, mesenchymal-epithelial transition factor; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer; PD-1, programmed cell death protein-1; PD-L1, programmed cell death ligand-1; PCR, polymerase chain reaction; ROS1, ROS proto-oncogene 1; RET, RET proto-oncogene; RT-PCR, real-time polymerase chain reaction; SCC, squamous cell carcinoma; SCLC, small cell lung cancer; T790M; substitution of threonine with methionine at position 790 of exon 20; TKI, tyrosine kinase inhibitor; TMB, tumour mutational burden; TTNA, transthoracic needle aspiration; TTNB, transthoracic core needle biopsy
Table 5 Summary of recommendations for molecular and biomarker testing in metastatic NSCLC in the Asia-Pacific region
- Adequate tumour tissue sample from the patient should be obtained for histological diagnosis and molecular testing at the time of diagnosis to guide selection for the most appropriate first-line treatment.
- Core tissue is the preferred tumour sample for molecular testing although cell blocks or cytopathology samples may also be tested if tissue sample is not available or not sufficient.
- EGFR mutation, ALK rearrangement and ROS1 rearrangement must be tested as standard of care for patients with metastatic adenocarcinoma who are being considered for an approved therapy targeting these molecular alterations.
- EGFR mutation, ALK rearrangement and ROS1 rearrangement may be tested in squamous cell carcinoma from small biopsies in never- (i.e., those who never smoked or smoked < 100 cigarettes in lifetime) or light- (< 15 pack-years) smokers or long-time ex-smoker (quitted > 10 years) and adenosquamous carcinoma to select patients for targeted therapy
- EGFR mutation test methods should cover common mutations in exons 18–21. At a minimum, when resources or tumour material are limited, exon 19 deletion and exon 21 L858R point mutation should be determined.
- IHC using a validated assay is comparable to FISH for ALK detection.
- IHC can be used to screen for ROS1; positive ROS1 IHC results should be confirmed by FISH or RT-PCR or NGS
- BRAF mutation status should be determined if BRAF/MEK inhibitors are available for use.
- Multiplexed genetic sequencing panels (e.g. NGS) are preferred over multiple single-gene tests to identify treatment options beyond EGFR, ALK, and ROS1. However, single-gene assays are still acceptable.
- New and emerging actionable biomarkers such as MET, RET, ERBB2 (HER2) and NRTK1 may be included in broad sequencing panels, if adequate material is available, to identify patients for treatment with specific inhibitors of these targets if such treatments are available or to direct patients to clinical trials.
- Compared to sequential single-gene testing, NGS targeted gene panel testing with its ability to test multiple genes of interest on limited material from small biopsies and cytological samples, reduces turnaround times, is more cost-efficient and sample preserving. EGFR, ALK, ROS1, BRAF, MET, HER2, RET and NTRK gene alterations may be tested using a NGS panel approach. However, in most clinical settings sequential testing may be more cost effective.
- Results of individual testing for EGFR mutation, ALK and ROS1 rearrangements should be available within 10 working days of receipt of sample, while results of broader molecular profiling by NGS should be available within 3 weeks.
- Molecular testing upon disease progression after first-line treatment should take into account the previous molecular testing results and therapies prescribed.
- In patients with EGFR-mutant NSCLC progressing on first- or second-generation EGFR-TKI, the detection of EGFR T790M acquired resistance mutation is essential to select patients for treatment with osimertinib, a third-generation EGFR-TKI effective against T790M-mutant.
- Plasma cfDNA testing is an acceptable approach to detect T790M at disease progression while on treatment with first- or second-generation EGFR-TKIs. If plasma testing is negative for T790M, tissue re-biopsy is strongly recommended to determine the T790M status and to exclude small cell transformation and other potential druggable resistance mechanisms.
- Testing at disease progression is not required for ALK-positive patients treated with crizotinib but may provide useful information to guide further treatment in patients treated with newer generation ALK-TKIs
- PD-L1 testing by IHC may be performed on FFPE tissue samples on a validated platform
- PD-L1 testing at baseline is recommended in patients whose tumours are negative for common oncogenic drivers (EGFR and BRAF mutations, ALK and ROS1 rearrangements) to select for anti-PD-1 or anti-PD-L1 therapy.
- PD-L1 testing is required for prescribing pembrolizumab therapy in all lines of treatment and not required but may be informative when nivolumab or atezolizumab are prescribed as monotherapy in the second-line setting.
Figure legend
FIGURE 1 Algorithm for molecular testing in metastatic NSCLC at initial diagnosis and at disease progression on first-line therapy
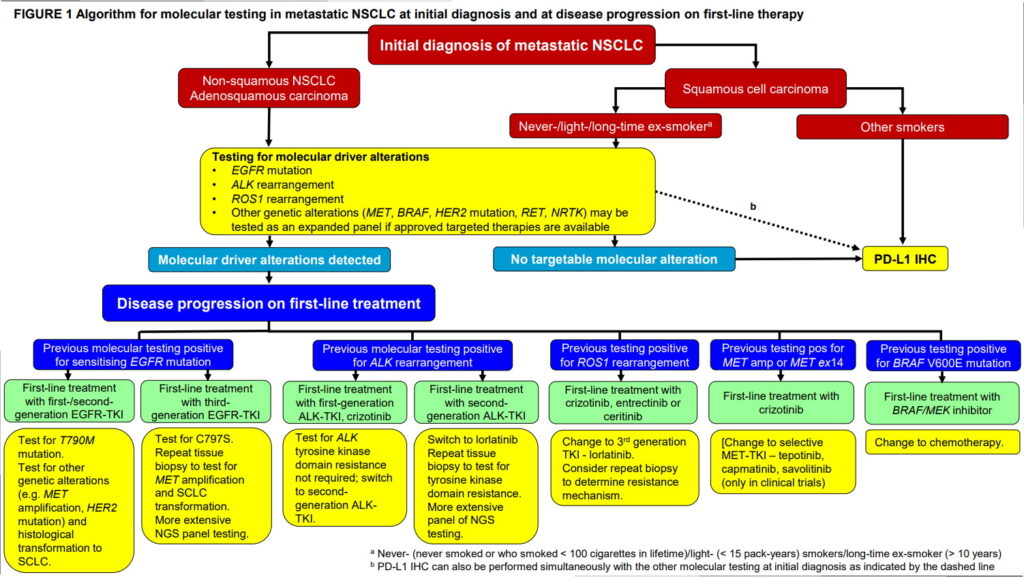
Abbreviations in Figure 1
ALK, anaplastic lymphoma kinase; BRAF, B-Raf proto-oncogene; C797S, substitution of cysteine with serine at position 797 of exon 20; EGFR, epidermal growth factor receptor; IHC, immunohistochemistry; MEK, mitogen-activated protein kinase kinase; MET, mesenchymal-epithelial transition factor; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer; PD-L1, programmed cell death ligand-1; ROS1, ROS proto-oncogene 1; RET, RET proto-oncogene; SCLC, small cell lung cancer; T790M, substitution of threonine with methionine at position 790 of exon 20;TKI, tyrosine kinase inhibitor
